
Comparative trial of Mhyosphere® PCV ID safety under field conditions in Canada
Porcine respiratory disease complex (PRDC) is a multifactorial disease resulting from the interaction of different infectious agents, management and environmental conditions, and is considered a major health concern. Mycoplasma hyopneumoniae (Mhyo) and Porcine Circovirus type 2 (PCV2) are among the main causes of PRDC1. Currently, control of these disease is mostly accomplished by improvement in management practices, good environmental conditions and immunizing prophylaxis by vaccination. These vaccines are mostly applied after weaning, a stressful and critical moment for piglet’s performance. Therefore, the safety of these vaccines could affect the performance of these animals after weaning.
Introduction
The aim of this study was to demonstrate the safety, through growth performance and mortality, of Mhyosphere® PCV ID and different Mhyo-PCV2 RTU vaccines under field conditions in Canada.
Materials and methods
A farrow-to-finish farm of 325 sows located in Canada positive of Mhyo, PCV2 and PRRS was selected. A total of 484 healthy piglets of 3 weeks of age from different parity sows were distributed in 4 different groups (day 0) taking into account gender and weight. At day 2, group A (n=121) was administered with 0.2 ml of Mhyosphere® PCV ID, an intradermal needle-free vaccine against Mhyo and PCV2 related diseases, all in one. Group B (n=121) was administered with 2 ml of a commercial intramuscular vaccine against Mhyo and PCV2, ready-to-use (RTU), Group C (n=121) was vaccinated with 2 ml of another different commercial intramuscular vaccine against Mhyo and PCV2, RTU, and group D (n=121) were pigs no vaccinated used as a control group.
The safety of each vaccine was measured through the weight of the pigs during the first 15 days. The pigs were weighed at day 9 and 15 of the study. The mortality was also recorded during these 15 days.
The statistical analysis was done by using R program.
Results
In order to know if there were significant differences of growth between the control non-vaccinated group and the different vaccines, a linear regression model was performed with vaccine and initial weight as factors.
Regarding the average daily weight gain (ADWG) between days 0-9, the initial weight was a significant factor as well as the vaccine. Considering the control group as the reference (Table 1), there was a significant and relevant reduction of ADWG with Vaccine B (-23.66 g/day), and relevant but not significant differences with Vaccine C (-16.70 g/day). Neither significant nor relevant differences with Mhyosphere® PCV ID (+2.51 g/day).
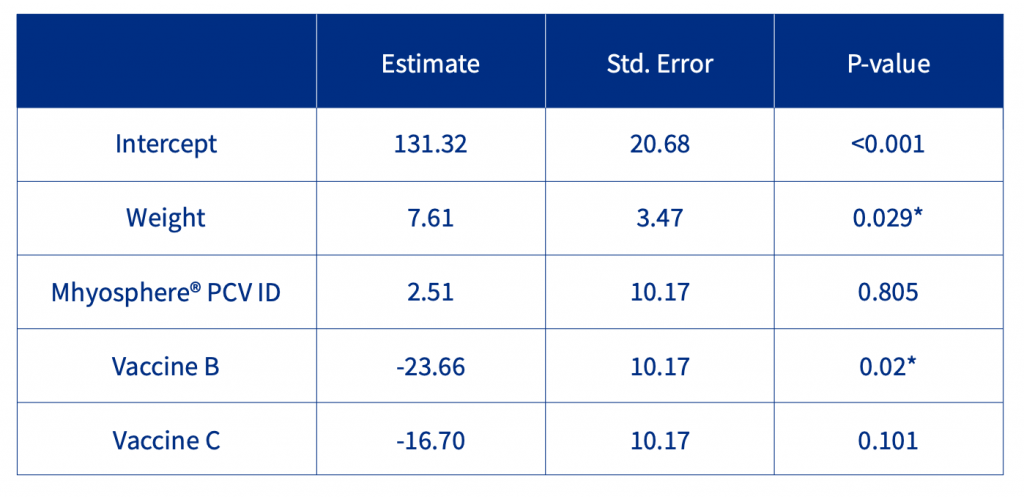
Table 1. Statistical analysis of ADWG during the first 9 days of the study.
Non-significant differences on ADWG (9-15 days) between groups were observed. Regarding mortality, there were no significant differences between groups during the studied period.
Discussion and Conclusion
Mhyosphere® PCV ID was the only vaccine without significant nor relevant differences on ADWG compared to the control group during the first 9 days after weaning; therefore, this vaccine demonstrated to be a solution to immunize against Mhyo and PCV2 related diseases without interfering with the growth of pigs during the entry into the nursery phase.
The reason behind the observed differences was not investigated in this study, however, it is known that the route of administration, intradermal, that has already demonstrated an advantage in terms of animal welfare2, but also the formulation of the vaccine (active substance and adjuvant) could influence the higher performance of piglets vaccinated with Mhyosphere® PCV ID.
References
1. Pieters M. and Maes D. Chapter Mycoplasmosis (863-883) Diseases of Swine, Eleventh Edition. Edited by Jeffrey J. Zimmerman et al. (2019)
2. A.Sanchez-Matamoros et al. 2021. Welfare effect of intradermal needle-free vaccination on piglets. ESPHM 2021. AWN-PP-08

