
Evaluation of ASF and PRRS virus transmission between pigs when using conventional needles and a needle-free device
Porcine reproductive and respiratory syndrome (PRRS), a devastating disease in pigs characterized by respiratory and reproductive disease, has been present in Southeast Asia (SEA) since the 1990’s1. In addition to PRRS, the spread of African swine fever (ASF) in the SEA region has increased the threat to ASF-free herds2. Intramuscular administration using needles has been the main route of vaccination in pigs although the risks associated with conventional needles are high. PRRS virus (PRRSV), for instance, was transmitted by conventional needles and was able to induce the disease in naïve pigs3.
Introduction
The objectives of this study were to evaluate African swine fever virus (ASF) and porcine reproductive and respiratory syndrome virus (PRRSV) transmission between pigs when using conventional needles and a needle-free device.
Materials and methods
In the present study, forty-two 3-week-old pigs were procured from a herd free of ASF and PRRSV. Their negative status against both pathogens was confirmed by PCR in blood samples upon arrival. Eighteen pigs were randomly allocated into 6 groups called seeders, of 3 pigs each, namely IM/ASF, ID/ASF, IM/PRRSV, ID/PRRSV and 2 control groups, NoChal/IM and NoChal/ID. Twenty-four age-matched pigs were divided into 4 groups of 6 pigs each as sentinels: IM/ASFsent, ID/ASFsent, IM/PRRSVsent and ID/PRRSVsent.
At 0 days post exposure (DPE), the IM/ASF and ID/ASF groups were exposed to ASF-infected pigs. The IM/PRRSV and ID/PRRSV groups were inoculated intranasally with 4 ml of HP-PRRSV-2 (106 TCID50/ml, 2 ml/nostril). At 7 DPE (0 days post injection (DPI)), the IM/ASF and IM/PRRSV groups were given 2 ml of a bivalent porcine circovirus type 2 (PCV2) and Mycoplasma hyopneumoniae (Mhyo) vaccine via the intramuscular route using conventional needles. The ID/ASF and ID/PRRSV groups were given 0.2 ml of a PCV2 and Mhyo vaccine (Mhyosphere® PCV ID, HIPRA) intradermally using a needle-free device (Hipradermic®, HIPRA). Also, at 7 DPE the same conventional needles and needle-free device were used to inject the same volume of the vaccine into the animals in the sentinel groups (1 exposed pig to 2 sentinels) with the same route of injection for each. Blood samples were collected from the seeders at 0, 7, 14, 21 and 28 DPE, and from the sentinels at 0, 7, 14, 21 and 28 DPI. ASF and PRRSV antibodies and viraemia were evaluated using ELISA and RT-qPCR respectively.
Results
The results demonstrated that the ASF and PRRSV seeder groups had the highest viraemia at 7 DPE. Following injection, sentinel pigs of the IM/ASFsent and IM/PRRSVsent groups were PCR positive at 7 DPI. In contrast, sentinel pigs of both the ID/ASFsent and ID/PRRSVsent groups were PCR negative throughout the experiment. Seroconversion results show that the IM/ASFsent and IM/PRRSsent groups had positive animals at 14 DPI, whilst the ID/ASFsent and ID/PRRSsent groups were negative throughout the experiment (Table 1).
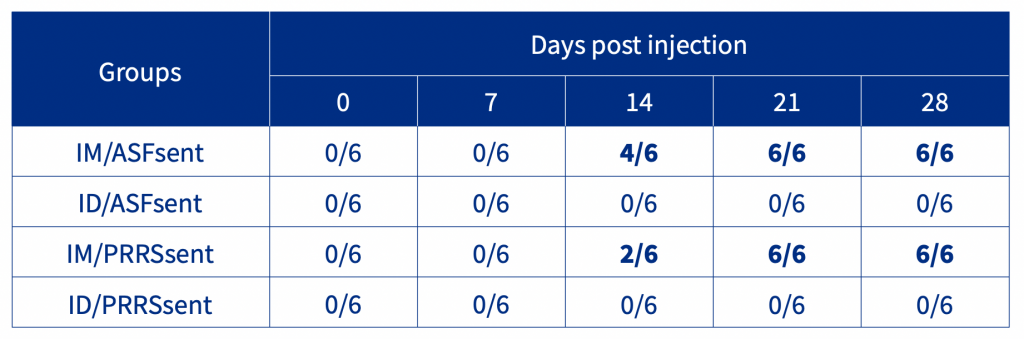
Table 1. Seroconversion of age-matched sentinel pigs following injection.
Discussion and Conclusion
Our findings revealed the potential for ASF and PRRSV transmission through needles during vaccination.
On the other hand, the possibility of applying intradermal vaccines with a needle-free device such as Hipradermic® inhibits both ASF and PRRSV transmission and could be used as an alternative vaccination route, avoiding iatrogenic transfer of pathogens between animals with shared needles.
Acknowledgments
This study was supported by Chulalongkorn University (Fundamental fund, grant number 65413100200013, CUFRB65_food(26)190_31_09).
References
1. Nilubol D et al. 2013. Genetic diversity of ORF5 gene of porcine reproductive and respiratory syndrome virus (PRRSV) genotypes I and II in Thailand. 158, 943-53.
2. Le V et al. 2019. Outbreak of African Swine Fever, Vietnam, 2019. 25, 1433-1435.
3. Madapong A et al. 2021 Safety of PRRSV-2 MLV vaccines administrated via the intramuscular or intradermal route and evaluation of PRRSV transmission upon needle-free and needle delivery. 11, 23107.

