
Mycoplasma hyopneumoniae is the causative agent of enzootic pneumonia (EP) and is one of the most prevalent and important agents associated with the porcine respiratory disease complex. EP is characterized by causing significant problems in the swine industry through endemicity, chronicity, decreased average daily gain, poor feed conversion, and increased days to market weight1,2. Another agent associated with the porcine respiratory disease complex is Porcine Circovirus type 2 (PCV2) which is characterized by clinical or subclinical infections among pigs. The most representative symptoms of the diseases include porcine dermatitis and nephropathy syndrome, which mainly occur during the growing or finishing stages of pigs; post-weaning multisystemic wasting syndrome, which affects nursery and growing pigs; and porcine respiratory disease complex (PRDC), which usually occurs in pigs 14–20 weeks of age 3,4. Both diseases are controlled by vaccination at weaning.
Introduction
The aim of this study was to evaluate the effect of intradermal needle-free vaccination on piglet performance and lung lesions at slaughter.
Materials and methods
A total of 1342 piglets, intact males and females, were individually weighed at weaning (21 days old), identified with ear tags and distributed between two groups according to sex and weight. Group 1 (n=671) was vaccinated with an intramuscular vaccine (IM-Competitor) against Mycoplasma hyopneumoniae and Porcine circovirus type 2, and group 2 (n=671) was vaccinated with Mhyosphere® PCV ID, an intradermal needle-free vaccine against Mycoplasma hyopneumoniae and Porcine circovirus type 2 associated diseases. The intradermal vaccine was administered with Hipradermic® 3.0. The body temperature was measured at 0 (time of vaccination), 6 and 24 hours after vaccination. All the piglets were individually weighed at 7 days and 42 days after weaning to assess weight gain in the nursery phase and at 180 days at the end of the finishing phase. At slaughter, the lungs were evaluated for the prevalence of macroscopic pneumonic lesions through the artificial intelligence application Hipralink Diagnos® (Hipra) with the MADEC modified system.
Results
The increase in body temperature after vaccination was affected by the administration route and vaccine used. The body temperature of the IM animals was 0.18 degrees higher than that of the ID animals in the first 6 hours after vaccination. The first week of nursery performance was affected by the vaccination, the IM animals had a lower Body weight [BW] and Average Daily Weight Gain [ADWG] than the ID piglets. No statistical differences were observed in the nursery phase, but in the finishing phase, the ID animals had a higher ADWG than the IM piglets, resulting in a 4.36 Kg increase in BW (Table 1). At slaughter, the ID animals had significantly (p<0.05, ordinal logistic regression) less lung lesions and a lower prevalence of pneumonic lesions than the IM animals (Figure 1).
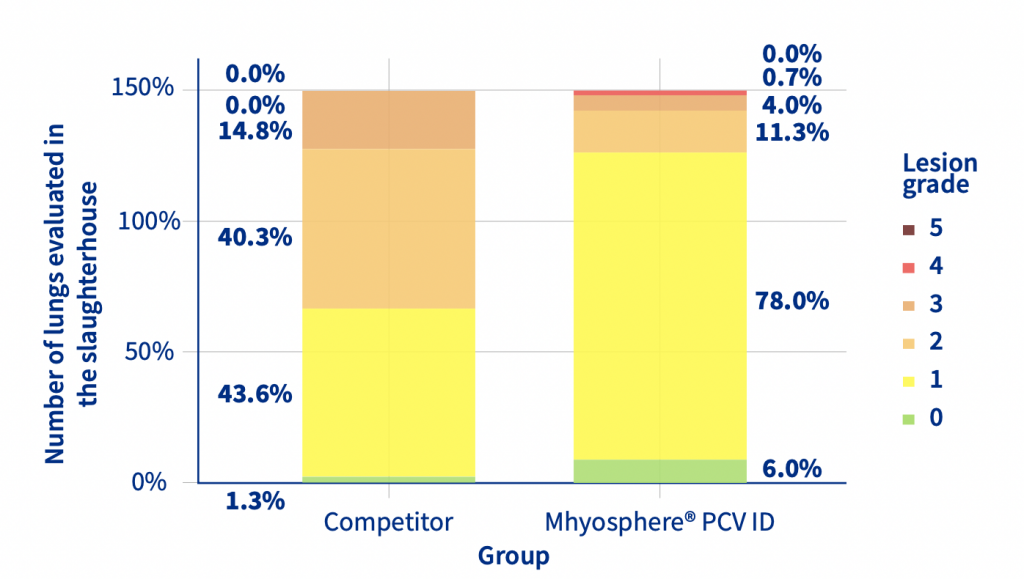
Figure 1. Prevalence of different grades of lung lesions between Mhyosphere® PCV ID (ID) and competitor (IM).
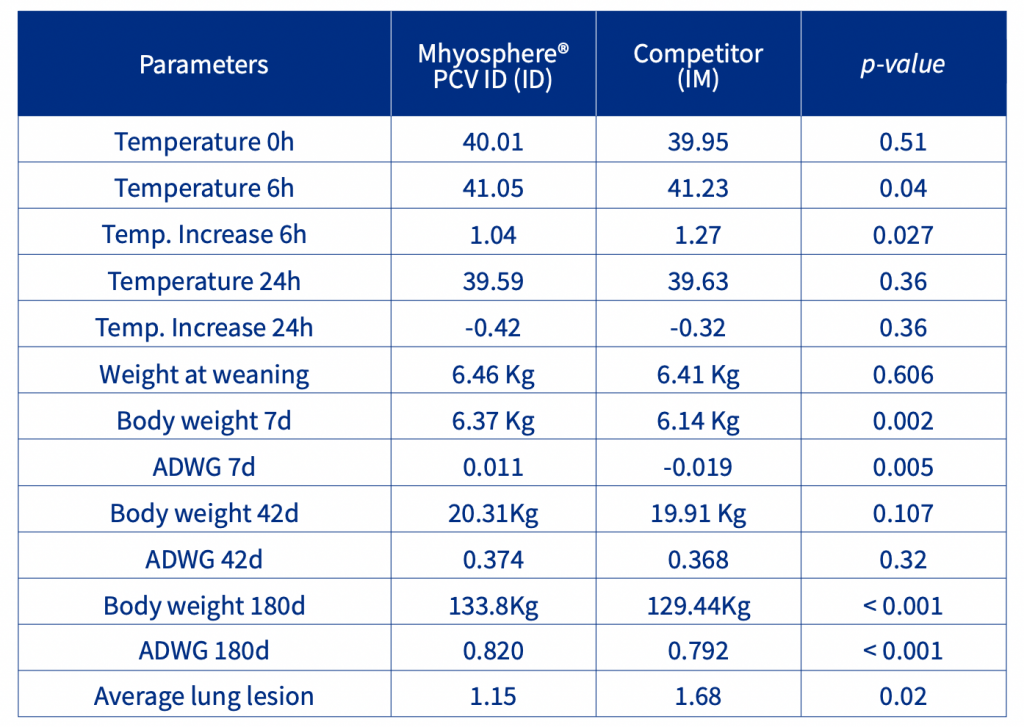
Table 1. Temperature and performance in the finishing phase and pneumonic lesions lung score.
Discussion and Conclusion
This study demonstrated that intradermal needle-free vaccination against Mycoplasma hyopneumoniae and Porcine circovirus type 2-associated disease with Mhyosphere® PCV ID is safe to administer in weaned piglets and effective in preventing losses caused by these agents, improving weight performance, and reducing pneumonic lung lesions.
References
1. Pieters MG & Maes D. 2019. Mycoplasmosis. In The Diseases of Swine, 11, 863-883.
2. Maes, D et al. 2018. Update on Mycoplasma hyopneumoniae infections in pigs: Knowledge gaps for improved disease control, 65 (1), 110-124.
3. Ren, L et al. 2016. Interactions of porcine circovirus 2 with its hosts, 52, 437-444.
4. Segales, J 2012. Porcine circovirus type 2 (pcv2) infections: Clinical signs, pathology and laboratory diagnosis, 164, 10-19.

