
Comparison of effects of Mhyosphere® PCV ID with three vaccine associations against Mycoplasma hyopneumoniae and PCV2 on piglet growth during nursery under field conditions
Ainhoa Puig 1, Ignacio Bernal 2, David Sabaté 1, Isaac Ballará 2, Jordi Montané 3, Lorena Nodar 4, Daniel Angelats 4 and Ramon Jordà 2.
Reduced version with authors’ approval. For full version please download the attached PDF.
Introduction
Porcine respiratory disease complex (PRDC) causes devastating economic losses to the swine industry due to reduced performance of the pigs, including a decreased growth rate and an increased feed conversion ratio, as well as an increase in the use of antimicrobials, mortality, and treatment costs (Ames 1999; Maes et al. 2003; Yang et al. 2020).
PRDC is a multifactorial disease resulting from the interaction of different infectious agents (viruses, mycoplasmas, and bacteria), management and environmental conditions, and host factors (Pallarés et al. 2021). M. hyopneumoniae (Mhyo) and Porcine circovirus type 2 (PCV2) are among the main causes of PRDC, of which Mhyo is a primary causing agent and PCV2 is considered secondary (Segalés et al. 2013). In the field, swine practitioners and producers use vaccination rather than antibiotic treatment and prefer to use single-dose vaccines against Mhyo and PCV2 to efficiently control PRDC (Hoelzer et al. 2018; Consortium members of work package 2 led by Andrea Ladinig, University of Veterinary Medicine, Vienna 2020; Yang et al. 2020). Mhyo and PCV2 vaccines may be combined in the field before their intramuscular administration, a process that might result in increased vaccination costs due to increased preparation times and possible vaccine mixing errors.

In addition to the inconveniences caused by the need of two separate vaccines to protect against Mhyo and PCV2, intramuscular injections using needles are considered painful and induce stress to piglets (Scollo et al. 2020; Temple et al. 2020; Dalmau et al. 2021). Furthermore, administration of two vaccines in association may cause increased pain. However, the use of needle-free devices for intradermal vaccination reduces vaccination-associated pain, resulting in decreased behavioral aversive responses and stress compared to conventional intramuscular injections, increasing animal welfare (Scollo et al. 2020; Temple et al. 2020; Dalmau et al. 2021). In this regard, the avoidance of invasive, stressful procedures has been associated with increased body weight gain (Morgan et al. 2019).
Among the different marketed vaccines, MHYOSPHERE® PCV ID consists of the inactivated recombinant M. hyopneumoniaecpPCV2 strain Nexhyon expressing the PCV2 capsid protein, forming a single active substance. This is the first vaccine administered intradermally using a needle-free device that protects from both agents simultaneously.
Despite the large number of studies assessing the efficacy of vaccination against Mhyo and PCV2, face-to-face safety comparisons of vaccine options under field conditions are missing, and the effects of the novel vaccine formulation and method of administration of MHYOSPHERE® PCV ID on the initial development of piglets compared to those of other commercially available vaccines remain unassessed. Given the stress associated with weaning and its detrimental effects on piglets’ health, it is essential to ensure safety and minimize stress during this period (Campbell et al. 2013). This study aimed to compare the effects of MHYOSPHERE® PCV ID when administered at weaning (approximately 3 weeks of age) with those of three different commercially available associations of vaccines under field conditions, using growth performance during the nursery period and incidence of adverse reactions after vaccine administration as welfare and safety parameters.
Materials and methods
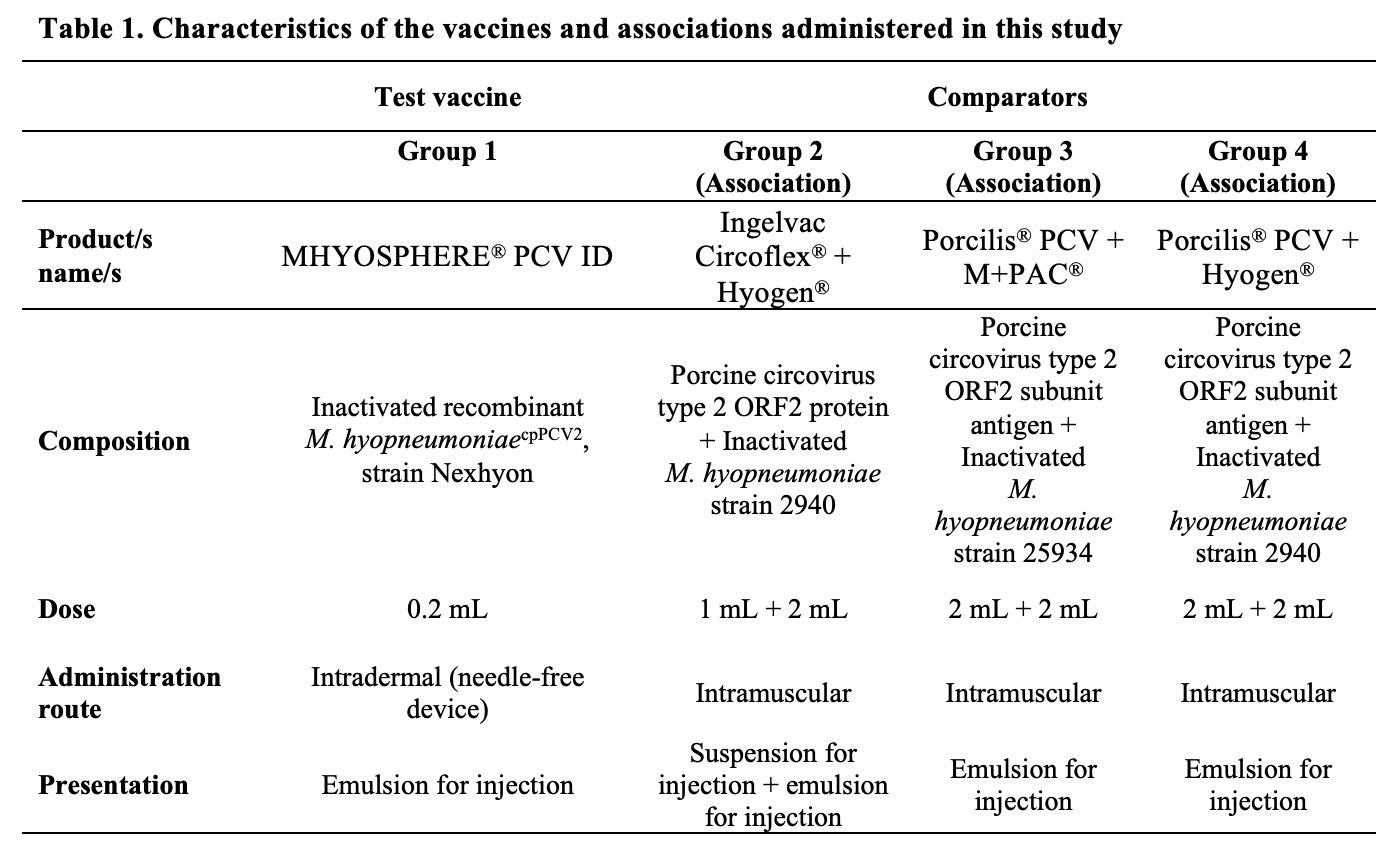
This was a randomized, blinded, controlled field trial including three-week-old clinically healthy piglets. The study was conducted in one commercial pig farm located in Spain with historical records of clinical and subclinical Mhyo and/or PCV2-related disease. Specifically, symptoms of PCV2 infection and the typical lung lesions caused by Mhyo infection had been reported in the past, and routine vaccination against PCV2 and Mhyo was implemented before this study started. Since the vaccination program started, both infections have been considered controlled. This farm has a continuous flow of piglets into the weaning unit in batches of 400–800 animals. Upon entrance to the nursery period at 21 days of age, piglets in each batch were randomized at 1:1 ratio based on stratification by covariates of interest (sex, genetics, and weight) to receive two treatments: test vaccine (Group 1 [G1]) or comparator vaccine (Groups 2, 3, or 4 [G2, G3, or G4, respectively]). Animals received the test vaccine in a single intradermal administration and each one of the three comparators in two different intramuscular injections in the neck area (one against Mhyo and one against PCV2).
Study Products
Industrial standard batches of the test vaccine MHYOSPHERE® PCV ID (HIPRA, Spain), G1; and the comparators Ingelvac Circoflex® (Boehringer Ingelheim, Germany) + Hyogen® (CEVA, France), G2; Porcilis® PCV (MSD, USA) + M+PAC® (MSD, USA), G3; and Porcilis® PCV + Hyogen®, G4, were administered at the doses indicated in the summary of product characteristics. The four vaccination approaches corresponding to each study group are described in Table 1. Furthermore, the PCV2 and Mhyo vaccine associations assessed are the most frequent in the global market. In this regard, Ingelvac® Circoflex is the reference and leader PCV2 vaccine on the market, and Hyogen® is considered the reference Mhyo vaccine on the market in Spain.
Clinical Safety Evaluation
The incidence of adverse reactions as well as the effect of vaccination on growth performance, used as a surrogate measure of animal welfare, were monitored during the study to evaluate the clinical safety of the test vaccine compared to the different vaccine associations. Different studies support a relationship between animal welfare and growth performance. In pigs, feeding patterns may be used as welfare indicators, and different stressors have been shown to impact growth performance (Martínez-Miró et al. 2016; Bus et al. 2021). Furthermore, a recent study showed that intramuscular vaccination was associated with lethargy and decreased feed intake after weaning, compared to intradermal vaccination, resulting in decreased growth performance during the nursery period (Bruna Ferrandin et al. 2022). Site staff responsible for safety evaluation observed the piglets immediately after vaccination and monitored them for two to four hours to record noticeable adverse reactions. Adverse systemic reactions considered were those not requiring pig handling, such as anaphylactic shock and vomiting, to preserve the field conditions and avoid additional manipulations causing piglet stress. Likewise, local adverse reactions, which may appear several hours after vaccination, were not monitored to minimize piglet manipulation. The live weight of pigs in each barnyard was measured at several time points between vaccination and the end of the nursery period (d35-d42 after vaccination). The duration of the nursery period ranged between 35 and 42 days for different batches. The average daily weight gain (ADWG; g/day) was calculated and analyzed over different periods: a) during the first 7 days and b) between the vaccination day (d0) and end of the nursery period. ADWG during the different periods was calculated as the difference between the starting and final weight divided by the duration of the stage. Data for dead or removed pigs were not excluded from the calculation due to logistic reasons.
Additional variables considered were pig’s batch (1 to 12), genetics (F2 and F7), sex, pen (A, B, and C), weight at entrance, which was categorized into 4 groups (< 4.78 kg, 4.78–5.6 kg, > 5.6–6.4 kg, and > 6.4 kg), and diarrhea (diarrhea during the first week and no diarrhea).
Results
Study Population
A total of 7072 21-day-old piglets (balanced number of males and females) from 12 consecutive weekly batches entering the nursery were randomly distributed into four groups: 3552 received the test vaccine MHYOSPHERE® PCV ID (G1); 1152 received the G2 control vaccine; 1143 received the G3 control vaccine; and 1225 received the G4 control vaccine (Figure 1). Mean (SD) body weight at entrance was 5.57 (1.17) kg; most animals (88.6%) had the F2 genotype and the remaining 11.4% the F7 genotype. The distribution of piglets in weight-at-entrance categories according to treatment group and comparison is shown in Figure S2 (Supplementary material).
Growth Performance
The multivariable regression analysis indicated that sex, pens, days of stay at nursery (35 or 42), and diarrhea were not associated with growth performance (p > 0.05). The genetic subgroup, weight at entrance, and batch variables were associated with growth performance and were therefore included in the final model (p < 0.05). The F2 genetic subgroup was the most frequent in this study (88.6%) and showed significantly increased ADWG at the end of the nursery period, compared to F7 genetic group (Figure S3). Likewise, increased weight at entrance was associated with higher growth performance (Figure S4). The variability in growth performance among batches was very high and was considered a random effect. Figure 2 displays analysis of growth performance of the F2 genetic subgroup according to batch and treatment group.
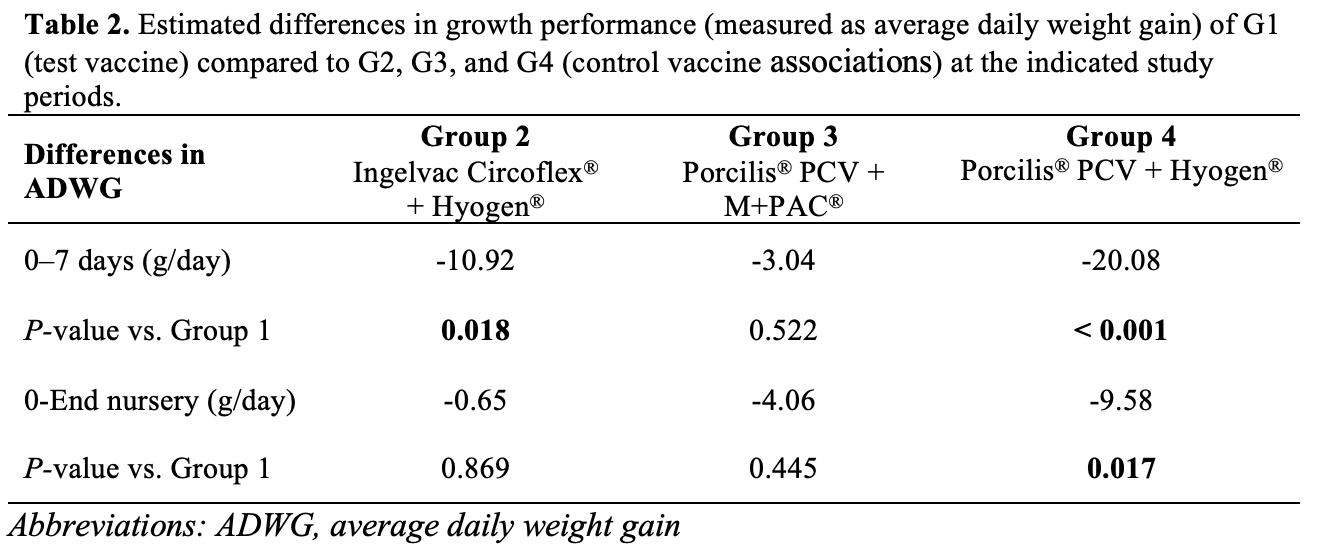
Results from the regression model, summarized in Table 2, showed that growth performance measured as ADWG (g/day) was higher in G1 than in G2, G3, and G4 during the first 7 days after vaccination, being the differences statistically significant when compared with G2 and G4. Similarly, growth performance at the end of the nursery period was higher in G1 than in G2, G3, and G4, being the differences statistically significant when compared with G4. Overall, ADWG for G1 was higher than for the other vaccine associations in both study periods.
Incidence of Adverse Reactions
Five animals from two different study groups experienced post-vaccination systemic reactions, one animal (0.03%) in G2 and 4 (0.32%) in G4, consisting of anaphylactic shock and vomiting. Specifically, one anaphylactic shock of mild severity was observed in one animal from batch 1 in G2 (0.37%). Two anaphylactic shocks were recorded in batch 4 of G4 (0.74%). Finally, one anaphylactic shock and one episode of vomiting were observed in batch 12 of G4 (0.52%). No adverse reaction was observed in G1 and G3.
Discussion and Conclusion
The present study assessed the effects of the vaccine MHYOSPHERE® PCV ID compared to those of three associations of commercially available vaccines against Mhyo and PCV2 infections under field conditions during the nursery period, by analyzing growth performance during the nursery period and incidence of adverse reactions after vaccination. Results from this study showed that piglets receiving the test vaccine (G1) had significantly higher growth performance compared to G2 and G4 during the first 7 days after vaccination, as well as significantly higher growth performance compared to G4 during the whole nursery period, without post-vaccination adverse reactions related to the vaccine.
The unique formulation and new active substance of M. hyopneumoniaecpPCV2 allows to reduce the administration volume to 0.2 mL, enabling the administration of the active substance by intradermal route using a needle-free device. Even though this study did not compare administration routes (intradermal vs. intramuscular administration), associations between intradermal administrations and increased animal welfare have been previously reported (Scollo et al. 2020; Temple et al. 2020). Temple et al. showed that piglets vaccinated intradermally (ID) had lower blood C-reactive protein and blood haptoglobin levels at 28 h post-vaccination compared to piglets vaccinated intramuscularly (IM), preventing the acute phase response and muscular damage associated with intramuscular injections (Temple et al. 2020). Sánchez-Matamoros et al. postulated that the extended times needed to cross the raceway and higher vocalization (presence and power) showed that IM injection was more aversive for piglets than ID injection (Sánchez-Matamoros et al. 2021).
In the present study, pigs receiving the control vaccine associations received two intramuscular injections. In this context, results from this study showing increased growth performance in pigs vaccinated with MHYOSPHERE® PCV ID may reflect a good health state (including the absence of negative experience, such as pain), suggesting a potential contribution to increased animal welfare. In this regard, a recent study showed that intramuscular vaccination was associated with lethargy and decreased feed intake after weaning, compared to intradermal vaccination, resulting in decreased growth performance during the nursery period (Bruna Ferrandin et al. 2022). Aside from the increased growth performance, intradermal vaccination using a needle-free technology has been shown to reduce transmission of PRRSV and other diseases, further contributing to controlling disease dissemination (Otake et al. 2002; Baker et al. 2012; Nilubol et al. 2022).
In conclusion, all the vaccination strategies assessed in this study were safe and did not induce any undesirable severe adverse reactions. Comparison of growth performance of piglets receiving the different vaccines showed that a single dose of MHYOSPHERE® PCV ID vaccine administered intradermally with a needle-free injector resulted in increased growth in the first seven days post-vaccination compared to vaccination comprising associations of single Mhyo and PCV2 vaccines. In addition, the ready-to-use M. hyopneumoniaecpPCV2 formulation in a single preparation and with only one active substance eliminates the disadvantages associated with the need to administer Mhyo and PCV2 vaccines in two independent IM injections. Given the increased growth performance and low adverse reactions shown in this study and the advantages of intradermal vaccination, the MHYOSPHERE® PCV ID vaccine stands out as a unique user-friendly and safe product for the porcine industry and, as shown in previous studies, contributes to controlling Mhyo and PCV2-associated diseases.
References
References available upon request or on the attached PDF.

